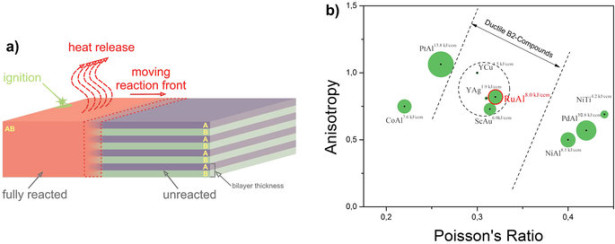
Established and already commercialized energetic materials, such as those based on Ni/Al for joining, lack the adequate combination of high energy density and ductile reaction products. To join components, this combination is required for mechanically reliable bonds. In addition to the improvement of existing technologies, expansion into new fields of application can also be anticipated which triggers the search for improved materials.
Here, we present a comprehensive characterization of the key parameters that enables us to classify the Ru/Al system as new reactive material among other energetic systems. We finally found that Ru/Al exhibits the unusual integration of high energy density and ductility. For example, we measured reaction front velocities up to 10.9 (±0.33) ms−1 and peak reaction temperatures of about 2000 °C indicating the elevated energy density. To our knowledge, such high temperatures have never been reported in experiments for metallic multilayers. In situ experiments show the synthesis of a single-phase B2-RuAl microstructure ensuring improved ductility. Molecular dynamics simulations corroborate the transformation behavior to RuAl. This study fundamentally characterizes a Ru/Al system and demonstrates its enhanced properties fulfilling the identification requirements of a novel nanoscaled energetic material.Nanometric reactive multilayers are capable of long-term chemical energy storage. The energy is released after local ignition during rapid self-propagating reactions (see reviews1,2,3). The reactions form intermetallic compounds within a micron-sized reaction front traveling along the multilayer (see Fig. 1(a)). This type of energy release makes reactive multilayers attractive as localized heat sources. Reactive multilayers of various chemistries have been explored to adjust the reaction properties to the requirements given by the application4,5,6,7,8,9,10,11,12,13. For example, in the NanoBondTM process, Ni/Al foil enables bonding of components at the smallest scale by building self-forming joints where only the joint interface exhibits heat-up. The need for external heat sources is eliminated, paving the way for accelerated production, e.g. in microelectromechanical systems (MEMS)13. However, the development of new joining strategies or the exploitation of novel applications requires three conflicting properties of the energetic systems14: 1.) reactions with maximal energy density (ED) leading to high reaction temperatures Tf, 2.) reactions to single-phase microstructures, and 3.) reaction products with room temperature (RT) ductility.
For more detail: Ru/Al Multilayers Integrate Maximum Energy Density and Ductility for Reactive Materials