The Norwegian explorer had set a new record for the closest approach to the North Pole, and now he was moving quickly over unbroken sea ice toward Cape Fligely and home. But then came a sickening realization: In his eagerness to break camp, he had forgotten to wind the chronometers. He had lost track of precise time, and thus the ability to track his longitude.
Although Nansen couldn’t have lost his position by more than a few minutes, it forced him to take a circuitously conservative route to avoid being swept into the North Atlantic. His expedition thus had to endure a hungry winter, camped on an unknown shore. Not until June the following year did he encounter other explorers and learn his true position—on Cape Felder, in Franz Josef Land.
Today, anyone with a smartphone can determine their time and position with ease. Satellites of the Global Positioning System (GPS) broadcast clock signals across the globe with uncertainties below 100 nanoseconds, or one ten-millionth of a second. These time signals carry the information needed for precise navigation: Because radio waves travel at exactly 0.299,792,458 meters per nanosecond (apart from minuscule variations due to refraction in the atmosphere), comparing signals from different satellites makes it possible to determine a position within a few meters. That’s why GPS has transformed seismic monitors, drone delivery, and many other applications.
But GPS can’t solve all timing problems. Central to the system are atomic clocks carried on each satellite. Although these clocks are extremely stable (and regularly calibrated by comparing them with ground-based atomic clocks at national standards laboratories), there are many ways to go wrong when transferring timing information to the user—jamming, spoofing, unintentional interference, solar storms, even reflections from buildings. But what if we could put this precision directly in the hands of the user by shrinking the atomic clock itself so it could work inside the GPS receiver? Would we, like Nansen, then want to carry our very best clocks with us?
In research now published at Physical Review Letters, we show that such a mobile clock is possible. We hope to make one soon.
The core of an atomic clock is a vacuum chamber containing a thin cloud of vaporized metal, usually cesium. Atoms in the vapor resonate at a precise frequency, meaning that their electrons will accept energy only from photons having just the right amount of it. If those photons have a little too much or too little energy—that is, if their frequency is a little too high or too low—the absorption falls off markedly. This is the key feature of an atomic clock.
Here’s how it works. An electrical oscillator creates a microwave frequency very close to the energy level of the atom we are using for our clock. If the oscillator deviates slightly from the correct frequency, the absorption changes, the change is detected by a laser, and the laser’s signal is used to tune the oscillator. This feedback loop corrects the oscillator’s imperfections.
Unlike the pendulum of a clock or the mechanical mechanism of a watch, atoms do not suffer from manufacturing error or wear; with proper isolation from the environment, their resonant frequency is set by the laws of physics. Achieving the necessary level of isolation in practice means that the best atomic clocks take up entire rooms. Commercial atomic clocks are usually the size of suitcases.
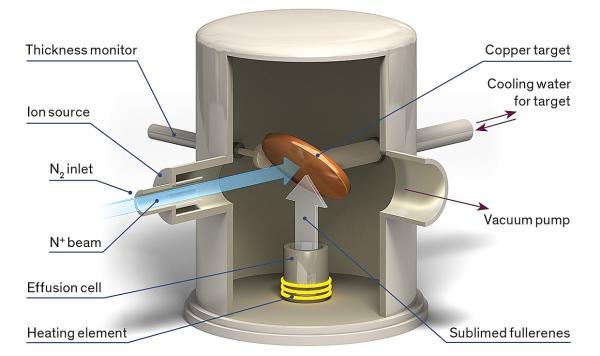
In 2004, in a tour de force of microfabrication, scientists at the National Institute of Standards and Technology managed to shrink this entire setup into a stack of components a few millimeters high. Such “chip-scale” atomic clocks are now available commercially and are used in niche applications, such as military communications and underwater navigation. But this miniaturization comes at a price—
Back to the atomic clock: We start with an oscillator that generates a radio signal close to the frequency that the nitrogen will absorb. We transmit the signal via an antenna to a cell containing a sample of the molecules, either as a powder or in solution. If the oscillator is correctly tuned, power is absorbed. If we see a reduction in the absorbed power, we’ll know that the oscillator has drifted away from the target frequency. Using a feedback mechanism, the oscillator can then be tuned back to the point of maximum absorption. Because this frequency is precisely known, an accurate time reference follows by simply counting cycles of the stabilized oscillator. We manage the feedback by modulating the oscillator frequency and having the detector look at that modulation. If the oscillator is set correctly, the modulation of the output is zero; if the oscillator’s central frequency has deviated, the sign of the output modulation tells us which side of the resonance it has moved to.
Endohedral fullerenes such as N@C60 are outstanding reference materials because, as we showed in 2006, the transitions between their quantum-mechanical spin states have some of the most precisely delineated frequencies of any molecule. If you draw a graph of the materials’ response to stimulating radiation, it will show a very narrow peak at the resonant frequency. Also, the fullerene cage prevents the walls of the container from affecting the frequency. One external influence does, however, penetrate the fullerene cage and can alter the relevant frequencies: a magnetic field. Because the world is full of uncontrolled magnetic fields—for example, from electric motors, steel vehicles, and Earth itself—protection from them is crucial for a stable clock. What Briggs and Ardavan realized is that for the N@C60 molecule, applying a small, static magnetic field can tune the energy levels in such a way that all magnetic influences on the resonance frequency cancel each other out.
The point, of course, is to one day incorporate a complete atomic clock into one chip. In this design, the entire operation is based on radio-frequency electronics, avoiding the need for optical elements, as used in conventional atomic clocks. And unlike a vapor-based clock, there would be no need to maintain a vacuum chamber and no power-hungry heater to drain a battery. An endofullerene-based atomic clock could thus be small, light, and energy efficient. Potentially, it could replace many of the quartz oscillators used in nearly every present-day electronic device to keep time.
Read More: To Build the World’s Smallest Atomic Clock, Trap a Nitrogen Atom in a Carbon Cage