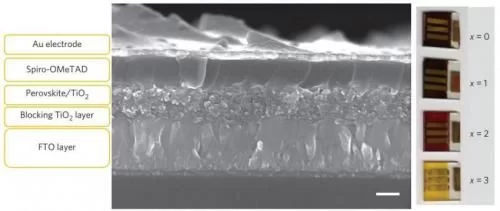
Northwestern University researchers are the first to develop a new solar cell with good efficiency that uses tin instead of lead perovskite as the harvester of light. The low-cost, environmentally friendly solar cell can be made easily using “bench” chemistry—no fancy equipment or hazardous materials.
“This is a breakthrough in taking the lead out of a very promising type of solar cell, called a perovskite,” said Mercouri G. Kanatzidis, an inorganic chemist with expertise in dealing with tin. “Tin is a very viable material, and we have shown the material does work as an efficient solar cell.”
Kanatzidis, who led the research, is the Charles E. and Emma H. Morrison Professor of Chemistry in the Weinberg College of Arts and Sciences.
The new solar cell uses a structure called a perovskite but with tin instead of lead as the light-absorbing material. Lead perovskite has achieved 15 percent efficiency, and tin perovskite should be able to match—and possibly surpass—that. Perovskite solar cells are being touted as the “next big thing in photovoltaics” and have reenergized the field.
Kanatzidis developed, synthesized and analyzed the material. He then turned to Northwestern collaborator and nanoscientist Robert P. H. Chang to help him engineer a solar cell that worked well.
“Our tin-based perovskite layer acts as an efficient sunlight absorber that is sandwiched between two electric charge transport layers for conducting electricity to the outside world,” said Chang, a professor of materials science and engineering at the McCormick School of Engineering and Applied Science.
Details of the lead-free solar cell will be published May 4 by the journal Nature Photonics. Kanatzidis and Chang are the two senior authors of the paper.
Their solid-state tin solar cell has an efficiency of just below 6 percent, which is a very good starting point, Kanatzidis said. Two things make the material special: it can absorb most of the visible light spectrum, and the perovskite salt can be dissolved, and it will reform upon solvent removal without heating.